Standard electron potentials (redox potentials) measure how easily a substance loses electrons. The more negative this value, the further the position of equilibrium is to the left.
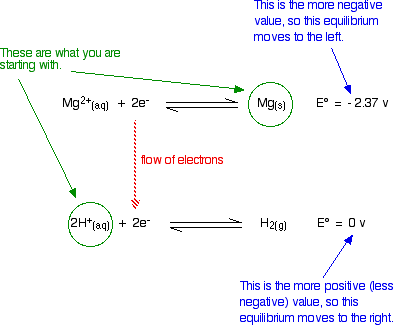
The Mg will freely turn into Mg ions, and will be able to give electrons to the hydrogen ions producing hydrogen gas.
http://www.chemguide.co.uk/physical/redoxeqia/predict.html

No comments:
Post a Comment