Vanadium (V)
Oxidation states: +5, +4, +3 and +2
Vanadium is usually found in ammonium metavanadate, NH4VO3. This is dissolved in sodium hydroxide solution (as it is not very soluble in water), and the solution can be reduced using zinc and an acid. The main ion present is VO2+, called the dioxovanadium(V) ion. V is reduced from +5 to +4.
Chromium (Cr)
Oxidation states: +6, +5, +4, +3, +2, +1, -1, -2
The simplest ion that chromium forms in solution is the hexaaquachromium(III) ion - [Cr(H2O)6]3+. It is fairly acidic and reacts with water in solution, where a hydrogen ion is lost from one of the ligand water molecules - it is acting as an acid.
Manganese (Mn)
Oxidation states: +2, +3, +4, +6 and +7
The simplest ion that manganese forms in solution is the hexaaquamanganese(II) ion - [Mn(H2O)6]2+. Hydroxide ions remove hydrogen ions from the water ligands attached to the manganese ion.
Ammonia acts as a base and removes hydrogen ions from the aqua complex.
Chemistry
Search This Blog
Tuesday, April 1, 2014
Saturday, March 29, 2014
Paramagnetism and Diamagnetism
Paramagnetism
Paramagnetism causes the substance to be attracted into the inducing magnetic field.
Paramagnetism is associated with unpaired electrons.
Any substance that has both paired and unpaired electrons will exhibit a net paramagnetism.
Diamagnetism
Diamagnetism causes the substance to be repelled into the inducing magnetic field.
Diamagnetism is associated with paired electrons.
Paramagnetism causes the substance to be attracted into the inducing magnetic field.
Paramagnetism is associated with unpaired electrons.
Any substance that has both paired and unpaired electrons will exhibit a net paramagnetism.
Diamagnetism
Diamagnetism causes the substance to be repelled into the inducing magnetic field.
Diamagnetism is associated with paired electrons.
Sunday, May 26, 2013
The Transition Elements: Types of ligand. Shapes and coordination numbers
Unidentate ligands: Ligands with only one donor atom, e.g. NH3, Cl-, F- etc.
Bidentate ligands: Ligands with two donor atoms, e.g. ethylenediamine, C2O42-(oxalate ion) etc.
Tridentate ligands: Ligands which have three donor atoms per ligand, e.g. (dien) diethyl triamine.
Hexadentate ligands: Ligands which have six donor atoms per ligand, e.g. EDTA.
Chelating Ligands: Multidentate ligand simultaneously co-ordinating to a metal ion through more than one site is called chelating ligand. These ligands produce a ring like structure called chelate. Chelation increases the stability of complex. This effect is called chelation effect.

The bond angles of the ligands around the central atom depends on the number of times it bonds.
http://www.askiitians.com/iit-jee-co-ordination-compounds/ligands-and-its-types/#
https://www.boundless.com/chemistry/transition-metals/coordination-compounds/coordination-number-ligands-and-geometries/
The Transition Elements: Formation of complex ions
A complex ion has a metal ion at its center with a number other molecules or ions surrounding it.
They are attached to the central ion by coordinate (dative covalent) bonds.
The molecules or ions surrounding the central metal ion are called ligands.
The active lone pairs of electrons in the outer energy shell are used to form coordinate bonds with the metal ion.
http://www.chemguide.co.uk/inorganic/transition/features.html
They are attached to the central ion by coordinate (dative covalent) bonds.
The molecules or ions surrounding the central metal ion are called ligands.
The active lone pairs of electrons in the outer energy shell are used to form coordinate bonds with the metal ion.
http://www.chemguide.co.uk/inorganic/transition/features.html
The Transition Elements: Catalytic properties. Laboratory and industrial examples.
Transition metal catalysts can either be homogeneous or heterogeneous catalysts (they can be in the same physical state as the reactants or not).
Laboratory example:
2H2O2 --MnO2--> 2H2O +O2
MnO2 is the catalyst.
Industrial example:
Transition metals can be used in petroleum and polymer (plastics, fibers) industries.
http://www.sky-web.pwp.blueyonder.co.uk/Science/transitionmetalcatalysts.htm
http://global.britannica.com/EBchecked/topic/602775/transition-element/81115/Transition-metal-catalysts
Laboratory example:
2H2O2 --MnO2--> 2H2O +O2
MnO2 is the catalyst.
Industrial example:
Transition metals can be used in petroleum and polymer (plastics, fibers) industries.
http://www.sky-web.pwp.blueyonder.co.uk/Science/transitionmetalcatalysts.htm
http://global.britannica.com/EBchecked/topic/602775/transition-element/81115/Transition-metal-catalysts
The Transition Elements: Explanation of color of compounds. The colours of the major ions
When transition metals form compounds, the d-orbitals of the metal interact with the electron cloud of the ligands in a way that the d-orbitals do not all have the same energy. The way they are split depends on the geometry of the complex. When the d-orbital is not completely filled, it is possible to promote an electron from a lower energy d-orbital to a higher energy d-orbital by absorbing a photon of electromagnetic radiation with the right amount of energy. The right amount of energy usually happens to be within the visible region of the electromagnetic spectrum. So, the light that isn't absorbed is what we see as the color of the compound.
http://www.wou.edu/las/physci/ch462/tmcolors.htm
http://en.wikipedia.org/wiki/Color_of_chemicals
| Name | Formula | Color | Picture |
|---|---|---|---|
| Copper(II) sulfate | CuSO4 | White | |
| Copper(II) sulfate pentahydrate | CuSO4 · 5H2O | Blue |  |
| Copper(II) benzoate | C14H10CuO4 | Blue |  |
| Cobalt(II) chloride | CoCl2 | Deep blue |  |
| Cobalt(II) chloride hexahydrate | CoCl2 · 6H2O | Deep magenta |  |
| Manganese(II) chloridetetrahydrate | MnCl2 · 4H2O | Pink |  |
| Copper(II) chloride dihydrate | CuCl2 · 2H2O | Blue-green |  |
| Nickel(II) chloride hexahydrate | NiCl2 · 6H2O | Green |  |
| Lead(II) iodide | PbI2 | Yellow |
http://www.wou.edu/las/physci/ch462/tmcolors.htm
http://en.wikipedia.org/wiki/Color_of_chemicals
The Transition Elements: Use of standard electrode potentials to predict reactions
Standard electron potentials (redox potentials) measure how easily a substance loses electrons. The more negative this value, the further the position of equilibrium is to the left.
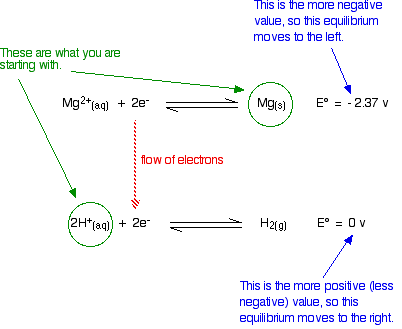
The Mg will freely turn into Mg ions, and will be able to give electrons to the hydrogen ions producing hydrogen gas.
http://www.chemguide.co.uk/physical/redoxeqia/predict.html

The Mg will freely turn into Mg ions, and will be able to give electrons to the hydrogen ions producing hydrogen gas.
http://www.chemguide.co.uk/physical/redoxeqia/predict.html
Subscribe to:
Posts (Atom)